Vom 20.09.2016 bis 20.12.2016 weilten 2 Gruppen à vier Studenten der Faculty of Biotechnology and Food Sciences of Lodz University of Technology”bei MITI zu einem studentischen Auslandseinsatz, gefördert von European Union within the European Social Fund, no. of financial support agreement: POWER.03.01.00-00-S084/15
Die fachliche Betreuung übernahmen Dr. Tatyana A. Karasyova und Prof. Dr. Edgar O. Klose.Im Folgnden der Abschlussbericht der Studenten.
I. THEORETICAL PART
- The description of the company.
Märkisches Institut für Technologie- und Innovationsförderung e.V. (MITI) was established on 26.06.2004 in the form of a registered association. Currently, it belongs to the 58 members of the country and abroad. The main objective of the work MITI lies in the natural sciences, and in particular the use of the results in a rural area. The starting point are the demands and problems of the Brandenburg. Conducted research, teaching and scientific support for the introduction and application of innovations in accordance with sustainable development and held for the benefit of man and nature. Cooperation Institute on the national and international level focusing on countries and regions struggling with the same or similar challenges as Brandenburg, in particular the neighboring Polish areas. - Short information about silicate compounds containing in zeolite. One of the greatest scientific and technological achievements of the 20th century is the discovery of the unique properties of zeolite. Zeolites are naturally occurring volcanic minerals which have formed over millions of years by the process of crystallization of volcanic ash submerged in the sea or fresh water lakes. It occurs in at least 50 countries of our planet, e.g. in Australia, Japan, Russia, Ukraine, USA, Spain, Latin America, Morocco, Turkey, Italy, Czech Republic, Slovakia, Bulgaria. The natural zeolite is chemically
a aluminum silicate, as much of our planet’s soils – clay, loam, loess. Natural zeolite contains 65 – 75% silicon and 10 – 12% aluminum. The ratio of the amounts of aluminum and silicon is 1:5 to 1:8. Zeolite is known for its curative, decontaminating and detoxifying effect for both humans and animals. It has a much stronger adsorption feature than charcoal and can neutralize toxins, pollutants, radionuclides and excess free radicals. Its air and water cleansing effects have been a topic of many international researches for the last 5 decades. It is used as soil additives to reduce the uptake of mercury by plants and restrict the entry of mercury into the food chain and are also used to remove ammonia from drinking water. Zeolite has one more very important feature, it is binding of the radionuclide radiations. This property has been successfully used to limit damages in several nuclear reactor accidents, e.g. Chernobyl (Ukraine), Fukushima (Japan), Harrisburg (USA). Experts are of the opinion that the daily intake of 5 – 6 g of natural zeolite powder is preventively the best protection against radioactive radiation. Zeolite to using for people, animals and plants is acquired with special micronization methods named TMAZ (Tribo Mechanically Activated Zeolite). - The products of company
The common basis of all products is a special form of Zeolite, the so called Klinoptilolit, that means a zeolite with a relation of silicon (Si) more than 80% and of Aluminium less than 20%. This is a “Conditio sine qua none”!!.

Figure 1. The products based on natural zeolite.Product nameMain advantagesProduct descriptionBiocaregreen Active Calcite®-100 % ecological -Fertilizing + protecting -Steady soil hydrology -Reinforcement towards -parasites -Rise in profits -Rise in quality Leaf fertilizer for benefiting plants and ornamental plants for spraying up on the leaves. Made of mineral calcite, which is of sediment origin. Biocaregreen Active Calcite® is
a powder. It will be sprayed on with a customary spray (e.g. backpack sprayer)Biocaregreen Natur Zeolith®(for domestic and farm animals) -100 % ecological -Better feed conversion -Faster growth -Longer laying performance -Reduction of the cel count in the milk of dairy cows -Reduction of mortality -Stress reduction in factory farming The German „Aiger-law” about antibiotic use will require extensive modifications of measures to encourage the maintenance of animal health by itself. The posibilities to get along in the present forms of rearing without medication, are limited. Therefore, we recommend Biocaregreen Natur Zeolith® for livestock.Biocaregreen Organik Aktiv®-PREMIUM plant aid -100 % ecological -Appeals very activating -Boosts the self protection -Protection against pests -Healthy fruit maturity and unusual flowering -Quality and yield-increasing Liquid plant excipient for useful plants and ornamental plants to spray on the leaves.Biocaregreen BIOreactor Air-ZKT 1,8-24h continuous operation (unattended operation) -Output 1.800 liters, every two days -Mobil plant on pallet -Heat pump operation -Production under aerobic conditions -Efficient and low energy use -Made Germany The reactor plant consists of
a reactor with 2 m net content and can establish a production volume of 1.8 m mineral solution within 48 hours. Over a circulation pump, the reactor content is mixed for
a uniform suspension deployment. The used heat pump cycle is monitored by temperaturę and pressure measuring instruments.
A discontinuous mineral component addition and dissolution of minerals used the setting of a stable pH value is possible. The filling and pumping of the system is automatic. The amptying of the reactor installation is done manually.
4. The philosophy of the device Plant Vital®PlantVital® is a system for measuring oxygen concentration including integrated temperature stabilization, with one, two or three measuring channels each consisting of an operating part and an oxygen sensor. The basic idea of PlantVital ® in 5000 exists in the supply of a technology, a measuring procedure and a measuring apparatus to the objective inquiry of the vitality (life ability) or the stress state of plants in terrestrial and in aquatic ecological systems which should mark the effect more of course and anthropogenic influences

in hand of quantitative parametersA part of a sheet (12 to 15 mm ²) is brought to the investigation of the desired object in a cuvette with an oxygen sensor in contact and is put out in a temperature-stabilized measuring chamber to different lighting relations. Besides, the oxygen concentration in the sample to be examined is registered over the sensor.
4.1. To the most important areas of PlantVital® system belong:-Optimisation of cultivation strategies under changed climate terms in agriculture and forestry. -Optimisation of the application of fertilizers and plant auxiliary materials, in particular with the transition from mineral fertilizer to organically engaged nutrients; optimisation of the application of residuals anaerobic digestion as a fertilizer. -Optimisation of the application of herbicides, pesticides and fungicides considering ecological principles. -Investigation of possibilities of the biological pest control. -Optimisation of food strategies in the “Third World” (choice of suitable genotypes – dry stress / crop). -Environmental monitoring, e.g., the propagation of pollutants from local sources (industrial objects) with the help of the vitality or stress state of widespread wild plants (indicator plants). -Student education in training periods and training courses.
4.2. Oxygen sensor.The sensor is used to determine the concentration of oxygen released during process of photosynthesis. Construction of the oxygen sensor
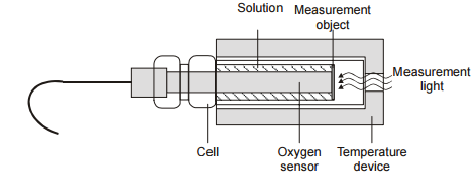
The advantages of the sensor: Miniature sensor structure Quick start operation High selectivity High stability Quick calibration on air (The sensor on the air gives a definite signal which must be related to the oxygen partial pressure of air under atmospheric pressure) Effective system integration
4.3. Operation of the deviceBefore starting the measurements you should do the calibration of the device. The sensor has to be calibrated on air. The calibration is monitored on the display of the computer.To use the device, follow these steps a) Cut off a definite piece of your plant using preferably the cutter in maintenance set.
b)By using the pipette give a drop of physiologic solution on the membrane of the sensor so the membrane is covered by the solution.

c)Place your piece of target on the sensors membrane that the stomata of the target face the membrane. Pay attention that no air bubbles remains between the target and the membrane.

d)Use the acryl cell screw it on the sensor’s crown carefully. Take care that the target remains symmetrically on the sensor’s membrane.

e)The button of the cell and the target on the membrane should reach a close contact.

f)Insert your prepared cell/target/sensor combination into the related by colour channel on the top of the device; the target is now in the channel with a stable temperature and can be illuminated by LED.

g)The „start will be executed using button START. On the monitor you can follow visually the target behaviour in the different phases of dark and light.
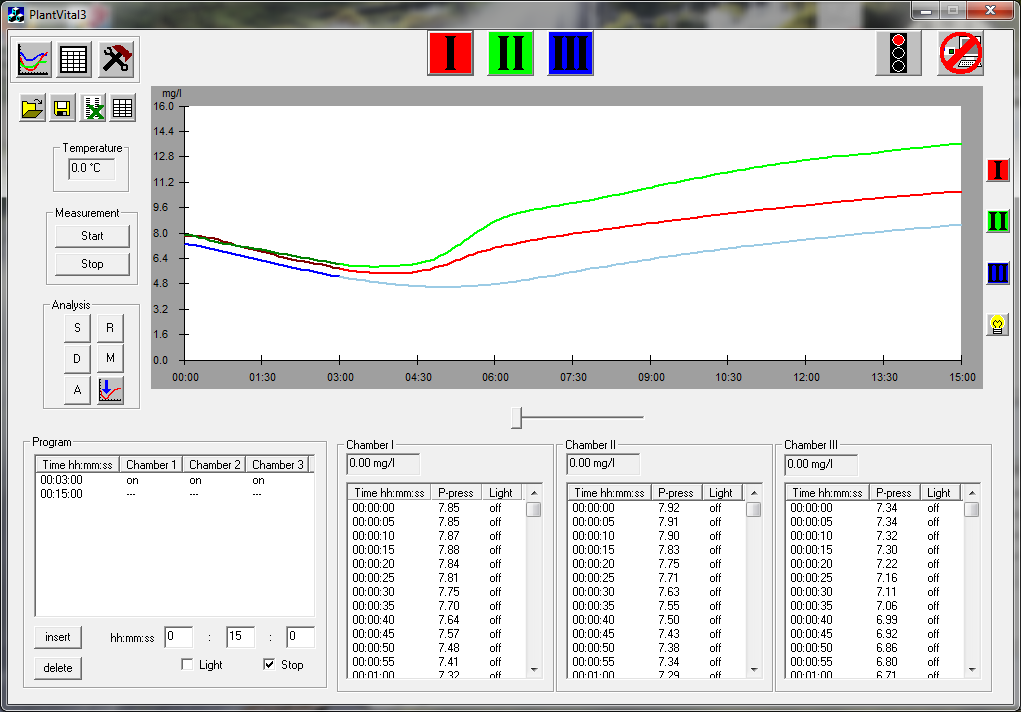
h)After that you can read the measuring parameters.
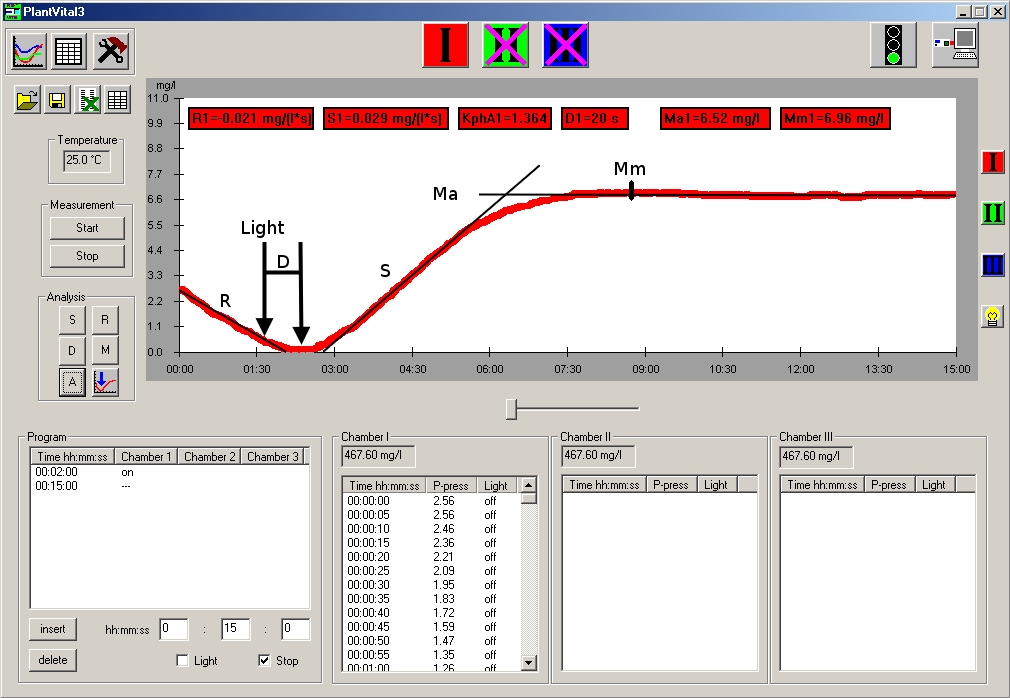
“R” – respiration; oxygen demand during “dark” (light independent) breathing“D” – time delay between switching light on and the minimum point of the curve (light compensation point)“S” – slope efficiency = release of oxygen during enzymatic photolysis“M” – maximum = equilibrium at the oxygen sensor“KphA” – S/R; „Photosynthetic activity coefficient“. This parameter is very useful in comparing the “Vitality Parameters” of the targets from different plant breeds.The parameters are determined automatically by the software. They are indicators for assessing the vitality and stress state of the plant sample. i)All results can be saved in the Exel program.
4.3.1. Test the functionalityOnce a month after sensor calibration you should check the functionality of the sensor. Measure the oxygen concentration in fresh tap water (poor in oxygen) by swinging slowly the sensor in the water. In this case a slight change in the oxygen partial pressure should be realised. This change in the partial pressure of more than 4% should be appear within 10 to 20 seconds. After taking of the sensor back to air and drying the membrane with care the value of the partial pressure should return to the value after calibration in the same period of time.
4.4. The advantages of using Plant Vital®-.Easy to handle and a very short time for probe preparation Quantitative measurement of the net oxygen release in the dark and under illumination. .Only small amount of material (a leaf, a coniferous needle, algae) is needed to get a reasonable signal. .Fully computerised, easy to run with all types of personal computers. .The temperature in the cell is always stable and can be selected. .Comfortable selection of temperature in the given range .Determination of selected quantitative values as a measure of the target‘s vitality. .Usage in the laboratory and in the field
4.5. In particular Plant Vital® is used in:High School education University student‘s training Research and development in plant breeding Prognostic assessment of plant development Water ecology and water management
- The comparison of three different methods to measure features of photosynthesis.
5.1. The photosynthesis process – a brief descriptionThe plant leaf absorbs light with a wide spectrum in the visible region. All parts of this spectrum can be absorbed by the plant according to its absorption spectrum. In the light harvesting system, the energy of all photons is transformed to light at 680 nm (photosynthesis system I) and 700 nm (photosynthesis system II). This photon energy can be used mainly in two different ways. 1)If there is no faster reaction this energy will be reemitted in form of fluorescence light. 2)The faster reaction is to create free electrons if this way is not closed. The photon energy is used to create electrons which will run along the electron transport chain to the place where the water molecules are split: 2 H2O → 4 H+ + O2The plant shall kick out the O2 in order to prevent a reaction with other molecules. The 4 protons are caught in less than 10-14 s by adenosine diphosphate to form adenosine triphosphate and by nicotine-amides to form a reduced version of nicotine-amides:NADH + H+ → NADH:H+Both NADH:H+ and adenosine triphosphate are highly energized molecules. The plant uses this stored energy to reduce CO2 to hexoses via trioses or tetroses (C3 – and C4-plants). To do that – to build up hexoses – the plant has to absorb and to store CO2.
5.2. Comparison of Three Different Methods to Measure Features of Photosynthesis
5.2.1. The fluorescence methodThe fluorescence measurement is a measure of all the electrons, which are released on PS II system and flow into the electron transport chain (ETC). However, not all the electrons contribute to primary production. Some of the electrons and the NADPH and ATP are eliminated by poising mechanisms, since under saturating light the light reaction provides more electrons than in the dark reaction (or in other metabolic reactions) are required. Some of these poising mechanisms (Mehler reaction, photorespiration) use the oxygen produced during the H2O slitting at PS II for other purposes.The fluorescence measurement is a measure of the electron transporting rate of the ETC. However, it is not apparent from it, how many of the transported electrons actually contribute to assimilatory processes and thus to primary production.
5.2.2. The CO2 – consumption methodOne brings a leaf in a chamber where the gas flow (input versus output) is measured. Looking for the CO2 – molecules on can assess how many CO2 molecules are absorbed by the plant in a given time from a given surface. Than we have got a parameter which indicates how many CO2 is in the leaf assuming that all the CO2 will be converted into hexoses. The CO2 measurement is a measure of the carbon fixation in the form of hexoses. However, the primary production includes not only the carbohydrate synthesis but also the protein and lipid synthesis. For the synthesis of these macromolecules, a considerable additional expenditure of energy and reducing equivalents is required. The CO2 measurement is a measure of the carbohydrate assimilation. But is not it the assimilation of other macromolecules can be seen.
5.2.3. The O2 measurement system using in PlantVital ® 5000In the case of PlantVital® 5000 one measures the amount of O2 in a given time at a given surface of the leaf (parameter “S”). Because after the “photolysis” of water, splitting H2O into 2H+ and O2, the living time of free H+ is shorter than 10-14s, there is no other way for H+ than to be caught by adenosine diphosphate and NADH and for the O2 molecules than to be kicked out of the cells and membranes.As a result the O2 measurement is the actual method for the estimation of vitality and stress state chlorophyll-bearing species.
II. EXPERIMENTAL
PART1 Practicing performing measurements Our first step in work with PlantVital® was to conduct experiments on wild, outdoor plants from Strausberg in order to practice performing measurements. The repeatability of measurements taken from one leaf can be influenced by different factors; the results of measurement can differ slightly depending on the part of the leaf from which the sample was cut, on the time which pass from the collection of the leaf to the measurement etc. In order to get results with standard deviation as small as possible, we needed to get acquainted with the impact of such factors. After conducting a few measurements we were able to get results which were significantly more repeatable than our first results.

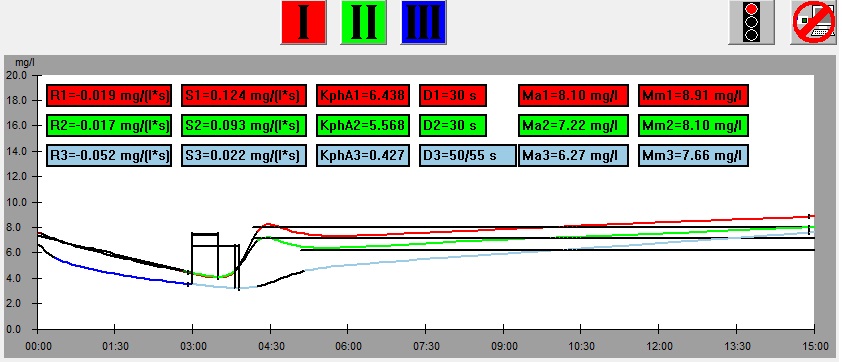
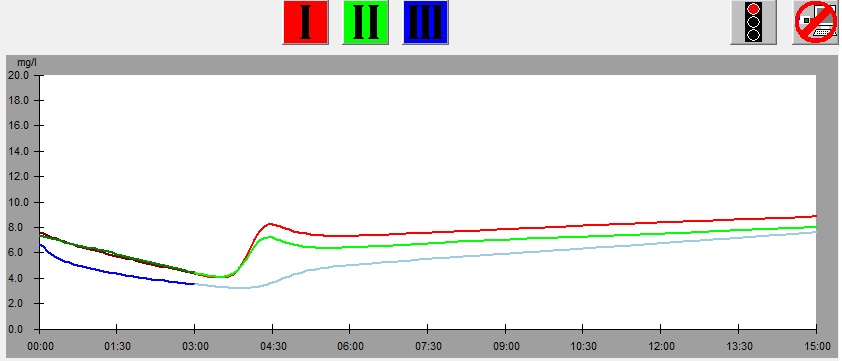
On the left there is a leaf from which samples was cut, below there is the graph showing results of the measurement of the leaf. At the bottom there is the same graph with parameters shown. The numbers on the picture of the leaf correspond to the numbers of measurements (1 – red, 2 – green, 3 – blue). This example illustrates dependence of the results of measurement on the part of a leaf from which the sample is taken.
2.The influence of ActiveCalcite® treatment on vitality of basil. The goal of the experiment was to evaluate the influence of ActiveCalcite® treatment on vitality of basil. The material for the experiment were two basil plants in pots, purchased in local store. Plant 1 was treated with ActiveCalcite®, plant 2 was not treated. The experiment lasted 7 days. Plants were purchased on 24th November, they were watered and measurements were performed on 24th, 25th, 28th, 29th and 30th November. Plant 1 was treated with ActiveCalcite® on 25th November. Than plants were not watered for two days. After that, on 28th November, both plants partly withered, condition of Plant 2 was significantly worse; its leaves were so withered that it was impossible to take measurements using PlantVital®. The next day, because of watering, the condition of both plants became better. It was possible to take measurement, also pictures of both plants was taken. Results of measurements, including parameters S (Slope) and KphA was shown on diagrams. Values shown on diagrams are averages of three measurement performed on every plant each day. Every three samples for measurement was cut from different leaves of the same plant.Left side: Plant 1, right side: plant 2.
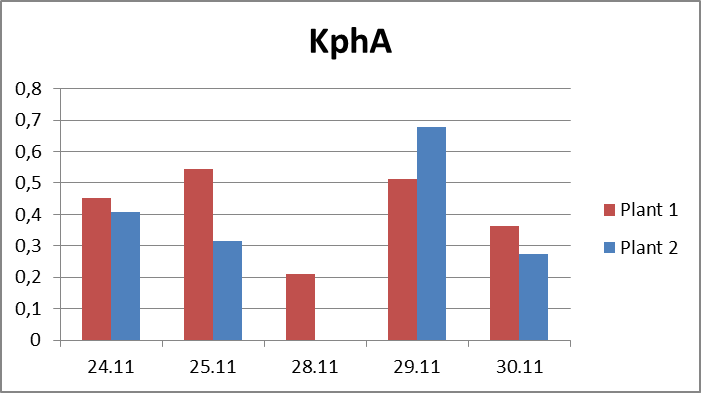
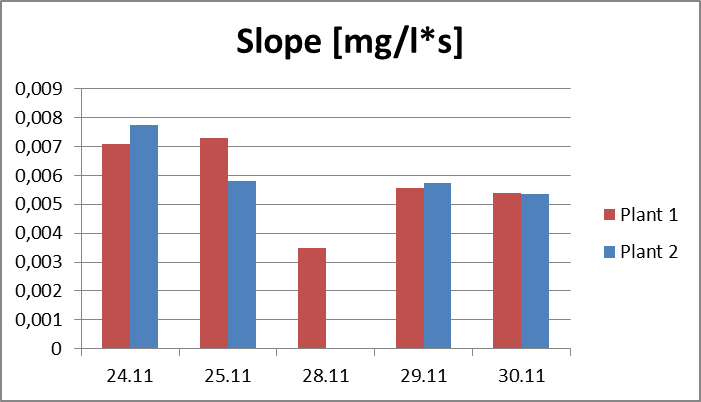
Pictures taken on 29th November.Discussion and conclusionAs was shown on pictures, after being exposed to drought stress and regaining vitality after watering, Plant 1 demonstrated significantly better condition than Plant 2. Some leaves of Plant 2 stayed withered after regaining vitality, and generally the whole plant looked less healthy. KphA parameters measured on 29th November indicates the opposite; it may be due to a measurement error. To conclude, the results of observation indicate that the ActiveCalcite® treatment may improve the plants resistance to drought stress and provide better vitality after it.
3.The influence of ActiveCalcite® treatment on plants resistance to drought stress. Research began on 11.16.2016 r., and was carried out in three variants: Plant 1: Cut-off part of a plant 3 placed in a bottle with water. Plant 2: Plant without the cut parts. Plant 3. Plant with the part cut off. On 16.11-21.11.2016r. plants were subjected to drought stress. In the days 21.11 – 25.11.2016 r. the plants were watered every day except for weekends. From the date of 06.12.2016 r. the plants were watered with aqueous solution of ActiveCalcite®. Measurements were performed on days 16.11., 18.11, 21.11, 23.11, 25.11, 06.12, 08.12 and 12.12.
The goal of the experiment was to evaluate influence of ActiveCalcite® treatment on the resistance of plants to drought stress. The way to collect and prepare data was analogical as in the previous experiment.Below there are pictures of Plant 1 (left side) and Plant 2 (right side). On the next page there are diagrams showing parameters S and KphA.
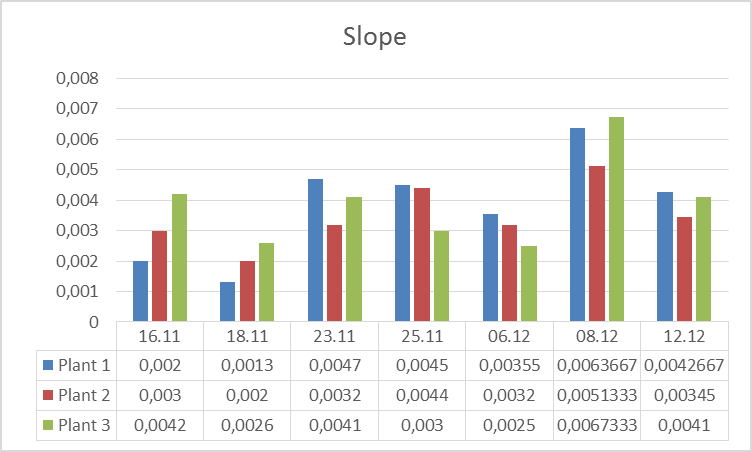
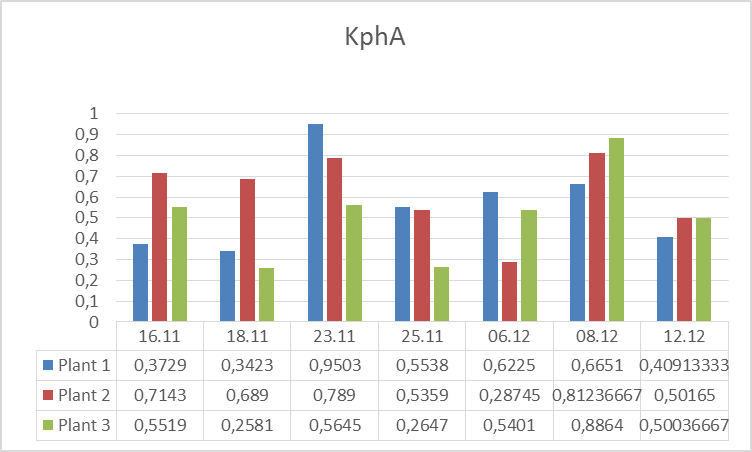
Discussion and conclusion From observation of plants it was impossible to perceive any difference in condition of different plants under different conditions they were subjected to. However, the results of measurements of vitality performed using PlantVital® indicate a significant decrease in vitality of Plant 1 subjected to drought stress in the beginning, an increase of vitality of all plants on 23.11 after watering, (for plants 1 and 2 the increase is significant) and an increase of vitality on 08.12 after watering with aqueous solution of ActiveCalcite® for both parameters. Taking the S parameter into consideration, the results indicate that the increase of vitality after treatment with aqueous solution of ActiveCalcite® (08.12) was more significant than the increase of vitality after using pure water (23.11) for all three plants.






